Curious and passionate readers, welcome to this new adventure of knowledge, where today we will delve into the complex and fascinating world of theoretical physics. In the panorama of this discipline, few theories provoke as much curiosity and debate as the paradox proposed by James Clerk Maxwell in his famous thought experiment of the Demon. In 1867, Maxwell presented a puzzle that challenged the second law of thermodynamics, questioning the principle of irreversibility of physical processes.
The heart of the experiment lies in a hypothetical being, Maxwell’s Demon, endowed with cognitive abilities that allow it to operate on individual gas molecules, separating them based on their kinetic energy. In this way, the Demon could theoretically create a temperature difference between two compartments without direct work or heat exchange with the surrounding environment.
In this article, we will explore the details of this fascinating paradox together, analyzing its implications and the responses it has generated in the scientific community. Happy reading!

Premise
The second law of thermodynamics, expressed through the definition of entropy S as a measure of the disorder or disorganization of a system, states that the total entropy of an isolated system can never decrease. In mathematical terms, for an isolated thermodynamic system, this principle can be formulated as follows:
![]()
where ![]() is the change in the total entropy of the system and the surrounding environment.
is the change in the total entropy of the system and the surrounding environment.
For an infinitesimal reversible process, the change in entropy ![]() is given by:
is given by:
![]()
where:
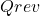 is the heat exchanged reversibly;
is the heat exchanged reversibly;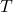 is the absolute temperature at which the heat exchange occurs;
is the absolute temperature at which the heat exchange occurs;
In a closed system that does not exchange matter with the environment, but can exchange energy in the form of heat and work, the change in entropy of the system is:
![]()
For irreversible processes, the total entropy of the universe (which includes the system and the environment) increases:
![]()
While for reversible processes, the total entropy remains constant:
![]()
Therefore, the second law of thermodynamics imposes that in any natural process, the total entropy of an isolated system cannot decrease, and can only increase or remain constant, thus reflecting the preferred direction of thermodynamic processes.
Role of Information and Information Theory
A crucial interpretative key is provided by information theory, especially through the contributions of James Clerk Maxwell and Claude Shannon. Maxwell’s Demon, in the act of discerning and separating molecules based on their energy, implies a process of collecting and using information. According to Landauer’s principle, the erasure of information by the Demon implies an increase in the entropy of the system, comparable to the dissipation of energy in the form of heat.
Mathematical Deepening and Conclusion
To quantify the thermodynamic cost of the Demon’s action, we can consider the work of Szilard and Bennett, who extended the concept of physical information and its relationship with thermodynamic entropy. Using concepts like Shannon’s information and information entropy theory, it is possible to demonstrate that the acquisition, processing, and erasure of information by the Demon inevitably require an energy expenditure that increases the overall entropy of the system.
James Clerk Maxwell, a pioneer of electromagnetic theory and thermodynamics, profoundly influenced scientific thinking not only with his work on gas kinetics but also with his ingenious thought experiment. His legacy has been further enriched by Claude Shannon, the father of information theory, who provided the mathematical foundations for understanding the flow and processing of information in physical systems.
In conclusion, although Maxwell’s Demon represents a formidable thought experiment that challenges conventional thermodynamic order, mathematical foundations and information theory confirm that the energy and entropy balance of the system remains intact. The unshakable validity of the second law of thermodynamics is maintained by the balance between information acquisition, processing, and the associated energy expenditure. Thus, Maxwell, with his ingenious conception, not only stimulates scientific debate but reaffirms the beauty and universality of the fundamental laws of physics.
